
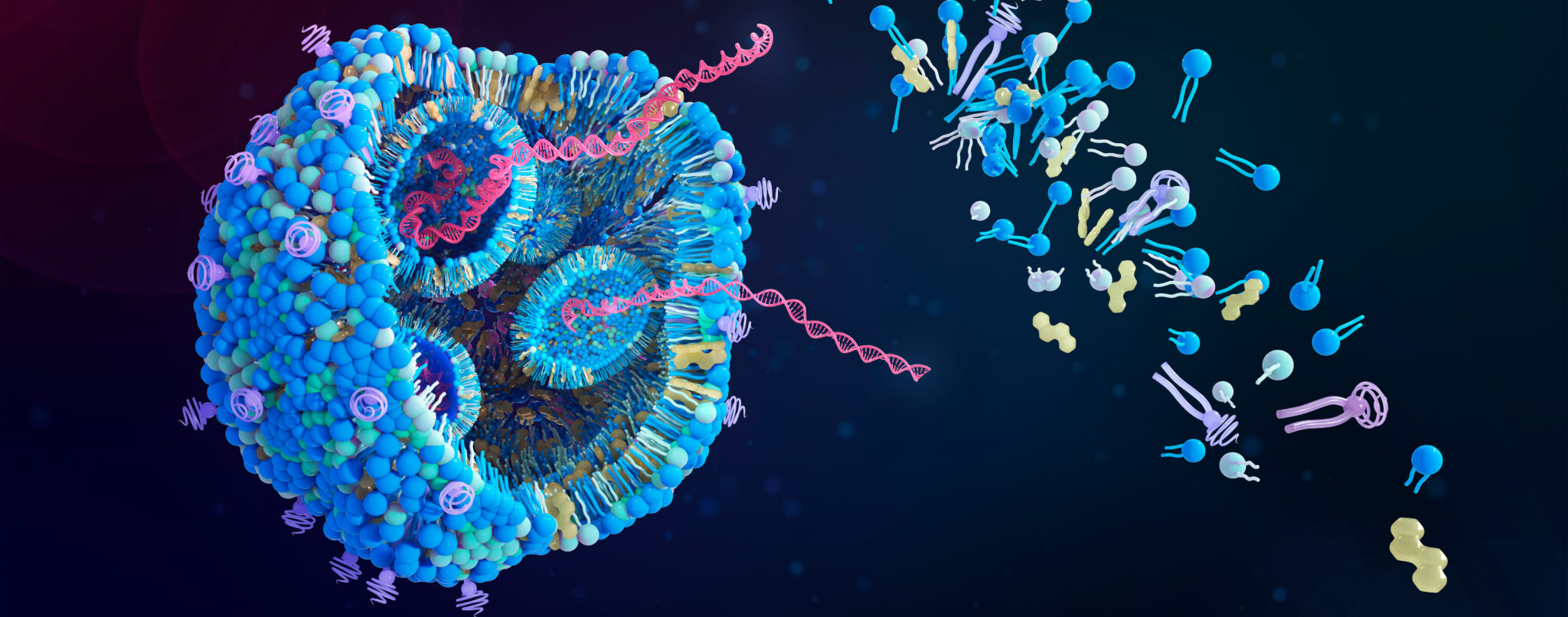

The era of protein therapeutics began in the 1980s with recombinant insulin, transforming modern medicine. Today, we are witnessing another groundbreaking shift with the rise of nucleic acid therapeutics (NAT). Advances in RNA biology and innovative delivery systems have unlocked powerful new treatment modalities, including antisense oligonucleotides (ASOs), small interfering RNAs (siRNAs), aptamers, and CRISPR-Cas9 gene-editing technologies.
The rapid development and global success of mRNA vaccines during the pandemic showcased the immense potential of mRNA as a drug substance and lipid nanoparticles (LNPs) as delivery vehicles.
This webinar will explore the exciting landscape of nucleic acid-based therapies currently making an impact in the clinic. We will highlight their structures, mechanisms of action, and regulatory approvals, while diving into the challenges and opportunities in drug product development. From the critical role of delivery systems like LNPs to the complexities of formulation and stability, we will explore why these aspects remain key hurdles for widespread adoption.
Examples of these hurdles are incomplete understanding of LNP structure-activity relationships and stability issues. Strategies to overcome them are available and include thorough analytical characterization and stabilization approaches like lyophilization. Finally, we will share case studies from Coriolis Pharma’s research that illustrate key stages of the drug product development roadmap and the associated analytical approaches required at each step.
Join us to gain insights into the science, technology, and innovation shaping the future of this rapidly evolving field.
By ticking this box, you agree to the privacy policies of Coriolis Pharma and Livestorm, consenting to receiving communications related to this topic and other products or services within their portfolio